Evaluation

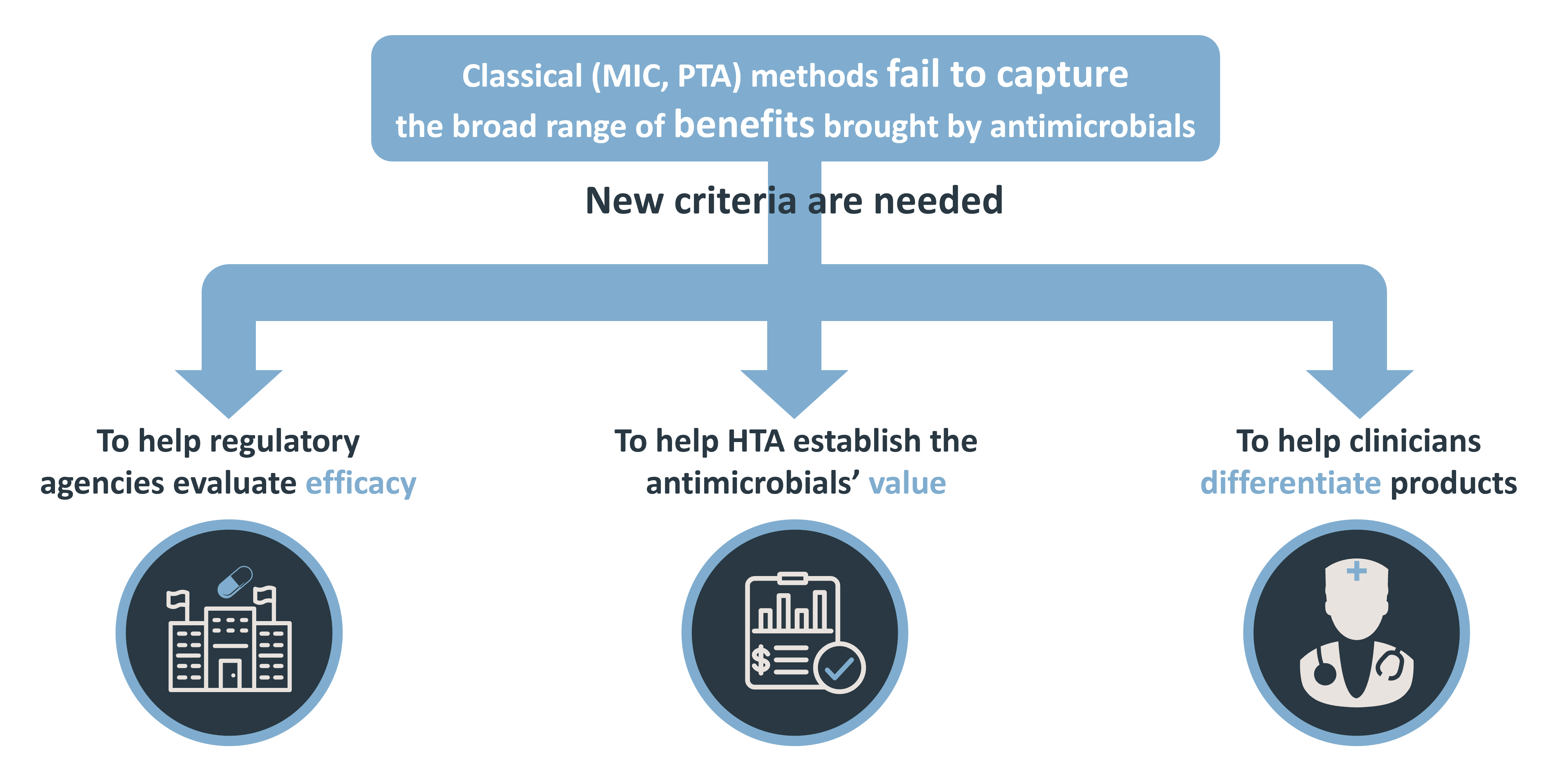
The efficacy of antimicrobials (ATMs) is traditionally measured using the minimum inhibitory concentration (MIC); the probability of target attainment (PTA) derived method is then used to assess clinical efficacy at a specific dosage for a specific MIC value. But the MIC and PTA methods alone can only measure certain pharmacological effects of an ATM, while many others are ignored. In these conditions, many ATMs are not evaluated at their true value, and it is difficult to differentiate between them.
We need to improve our ATM assessment methods, in particular by introducing new criteria that are better able to consider all the pharmacological effects that an ATM can generate.
Our Approach
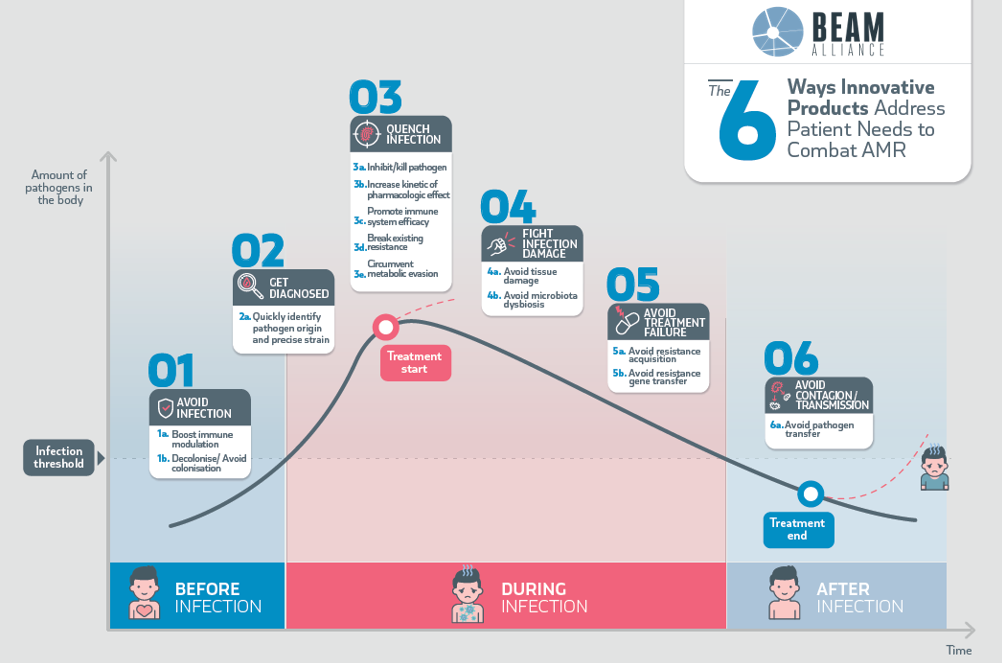
Based on the pathogenesis of infectious diseases, the BEAM Taskforce on Evaluation (BTF-E) has proposed a six-way approach to characterising ATM activity. The corresponding BEAM Infection Curve (below) serves as a roadmap for the identification and validation of new clinical endpoints.
Since then, constructive work has been undertaken with key stakeholders (EMA, EUCAST, FDA, CLSI, etc.) to improve the regulatory framework.
BTF-E is freely accessible to all BEAM members.