Pipeline

The pipeline of antimicrobial products under development is thin. But innovative.
Indeed, the antimicrobial products under development display a variety of pharmacological effects and applications.
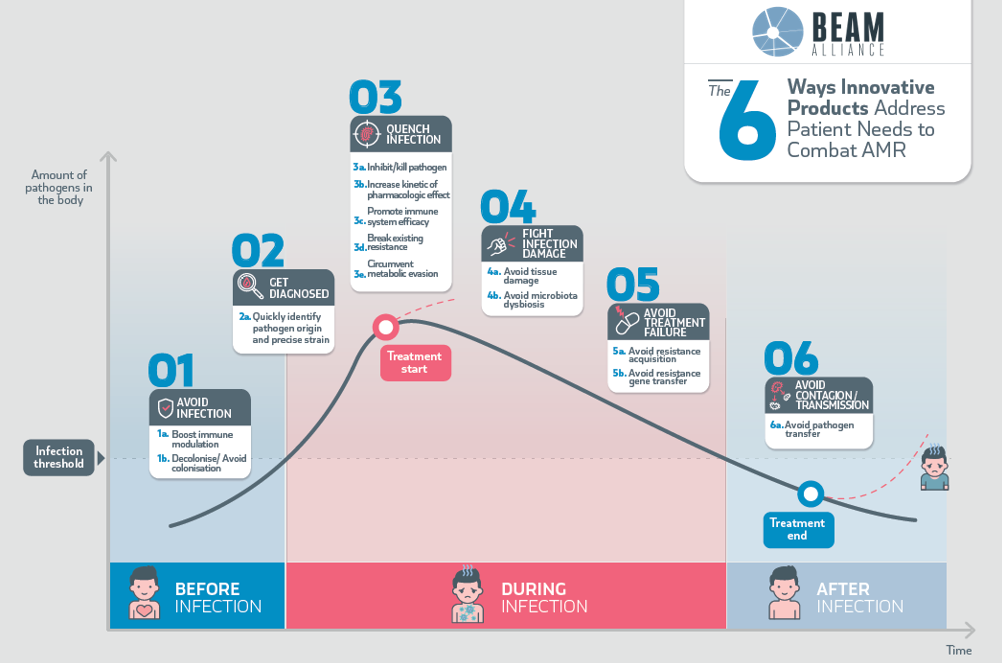
They are described in the BEAM Infection Curve (BIC); read the memo to find out more.
Take a look at all these products that could save lives in the future.
Pipeline RX
Click on column headers to sort the table
| member | product | development stage | |||
|---|---|---|---|---|---|
| Telum Therapeutics | EpleTTX4 | Discovery | |||
| Telum Therapeutics | EpleTTX3 | Lead opt | |||
| Telum Therapeutics | EpleTTX2 | Pre-clinical | |||
| Telum Therapeutics | EpleTTX1 | Pre-clinical | |||
| Ilya Pharma | ILP101-Inhale | Pre-clinical | |||
| Ilya Pharma | ILP100-Oral | IND/CTA ready | |||
| Ilya Pharma | ILP100-Topical | Phase III | |||
| Basilea Pharmaceutica International | BAL2062 | Phase I | |||
| Basilea Pharmaceutica International | Fosmanogepix | Phase II | |||
| Basilea Pharmaceutica International | Fosmanogepix | Phase III | |||
| Basilea Pharmaceutica International | Cresemba® isavuconazole | Marketed | |||
| Basilea Pharmaceutica International | Cresemba® isavuconazole | Marketed | |||
| Eligo Bioscience | EB003 | IND/CTA ready | |||
| OM Pharma | Uro-Vaxom® | Marketed | |||
| OM Pharma | Broncho-Vaxom® (Bacterial Lysates – OM-85) | Marketed | |||
| OM Pharma | OM-85 | Phase II | |||
| OM Pharma | OM-89 | Phase I | |||
| OM Pharma | OM-85 | Phase I | |||
| DevsHealth | Multi-resistant s. aureus novel treatment | Discovery | |||
| ArrePath | AP-001 | Lead opt | |||
| Tabrix | anti-virulence agents | IND/CTA ready | |||
| Granulytics | Undisclosed | Pre-clinical | |||
| Santero Therapeutics | SAN-Sa1 | Pre-clinical | |||
| Ultupharma | ULT1 | IND/CTA ready | |||
| Ultupharma | ULT2C | IND/CTA ready | |||
| Ultupharma | ULT2B | IND/CTA ready | |||
| Ultupharma | ULT2A | IND/CTA ready | |||
| Ultupharma | ULT3 | IND/CTA ready | |||
| VAXDYN | AcinetoVax | Pre-clinical | |||
| VAXDYN | EcoVax | Pre-clinical | |||
| VAXDYN | P-Vax | Pre-clinical | |||
| VAXDYN | K-Vax | IND/CTA ready | |||
| Arivin therapeutics | Viri-2425 | Pre-clinical | |||
| Arivin therapeutics | Viri-01 | Pre-clinical | |||
| Thioredoxin Systems | EbsArgent | IND/CTA ready | |||
| BioNTech R&D | BNT331 | IND/CTA ready | |||
| Basilea Pharmaceutica International | Tonabacase | Phase I | |||
| Basilea Pharmaceutica International | BAL2420 (LptA inhibitor) | Pre-clinical | |||
| Nexbiome Therapeutics | BGA-1901 | Pre-clinical | |||
| Nexbiome Therapeutics | BGY-1601 | IND/CTA ready | |||
| Nexbiome Therapeutics | NIN-2101 | Lead opt | |||
| SurvivX | SUR-101 | IND/CTA ready | |||
| Infex Therapeutics | GON-X | Lead opt | |||
| Infex Therapeutics | VAP-X | Lead opt | |||
| Disperazol Pharma | Disperazol | Pre-clinical | |||
| Disperazol Pharma | Disperazol | Pre-clinical | |||
| Osta Therapeutics | Osta 27 | Pre-clinical | |||
| AUROBAC THERAPEUTICS | ATX401 | Pre-clinical | |||
| AUROBAC THERAPEUTICS | ATX101 | Pre-clinical | |||
| NanoReviv | Staph-EX | Pre-clinical | |||
| discoveric bio beta | NIDB-3002 | Lead opt | |||
| discoveric bio beta | NIDB-3001 | Lead opt | |||
| Omnix Medical | OMN71 | Pre-clinical | |||
| Omnix Medical | OMN51 | Pre-clinical | |||
| XF-73 | Pre-clinical | ||||
| XF-73 | Pre-clinical | ||||
| XF-73 | Pre-clinical | ||||
| XF-73 | Pre-clinical | ||||
| XF-73 | Phase II | ||||
| NTCD-M3 | Phase II | ||||
| SPOR-COV | Pre-clinical | ||||
| Infex Therapeutics | RESP-X | Phase II | |||
| Infex Therapeutics | MET-X | IND/CTA ready | |||
| Infex Therapeutics | RDX-02 | Lead opt | |||
| AdjuTec Pharma | APC247 | IND/CTA ready | |||
| F2G | Olorofim | Phase III | |||
| AdjuTec Pharma | APC148 | Phase I | |||
| OLGRAM | OL2001 | Pre-clinical | |||
| Selmod | slm 500 | IND/CTA ready | |||
| Selmod | slm 300 | Lead opt | |||
| Selmod | slm 100 | Lead opt | |||
| Assuré Medical | Sol-UTI | Pre-clinical | |||
| BioVersys | BV100 | Phase II | |||
| ANTABIO | SBLi (ANT3310) | IND/CTA ready | |||
| VibioSphen | VIKYNG | IND/CTA ready | |||
| QureTech Bio | GmP | Lead opt | |||
| QureTech Bio | MTI | Lead opt | |||
| Omnix Medical | OMN6 | Phase I | |||
| Northern Antibiotics | NAB815 | Lead opt | |||
| Northern Antibiotics | NAB739 | Lead opt | |||
| tamrisa | 2G-DAB | Lead opt | |||
| tamrisa | EBL-1463 | Pre-clinical | |||
| IMMUNETHEP | UNImAb | Lead opt | |||
| IMMUNETHEP | PNV | IND/CTA ready | |||
| Hypharm | HY-133 | Phase I | |||
| Eligo Bioscience | EB004 | Pre-clinical | |||
| Debiopharm International | Debio 1453 | IND/CTA ready | |||
| Debiopharm International | Afabicin | Phase II | |||
| Combioxin | CAL02 | Phase II | |||
| Centauri Therapeutics | ABX01 | Pre-clinical | |||
| Centauri Therapeutics | ABX02 | Pre-clinical | |||
| BioVersys | BV200 | Lead opt | |||
| BioVersys | BV500 | Lead opt | |||
| BioVersys | alpibectir (BVL-GSK098) | Phase II | |||
| Basilea Pharmaceutica International | Zevtera® ceftobiprole | Marketed | |||
| Basilea Pharmaceutica International | Zevtera® ceftobiprole | Marketed | |||
| ANTABIO | PEi (ANT3273) | Pre-clinical | |||
| ANTABIO | MBLi (ANT2681) | IND/CTA ready | |||
| ALLECRA THERAPEUTICS | Cefepime / Enmetazobactam | Phase III | |||
| Akthelia | AKT-011 | Pre-clinical | |||
| Page /10 | |||||
*BIC: BEAM Infection Curve
The displayed information are not necessarily reflecting the official current position of each BEAM members; please refer to each company’s website for updated information, or contact them directly.
Pipeline DX
Click on column headers to sort the table
| member | kit/test | development stage | |||
|---|---|---|---|---|---|
| Dermatophyte LFA | Prototype in Lab | ||||
| Ocular fungal infection | Prototype in Lab | ||||
| Ocular fungal infection | Feasibility Phase | ||||
| CocciID | Feasibility Phase | ||||
| HistoID | Development Phase | ||||
| MucorID | Development Phase | ||||
| Microbira | MAAP-IR | CE-IVD and/or FDA 510k | |||
| Microbira | Microbira Prototype | Prototype in Lab | |||
| Microbira | Microbira Prototype | Prototype in Lab | |||
| Nostics | DUTI | Feasibility Phase | |||
| Molsid | fMic | Feasibility Phase | |||
| Molsid | Pythia UTI | Development Phase | |||
| Molsid | Pythia Staph | Development Phase | |||
| Page /2 | |||||
The displayed information are not necessarily reflecting the official current position of each BEAM members; please refer to each company’s website for updated information, or contact them directly.