Phages
Phage Therapy and phage derived products
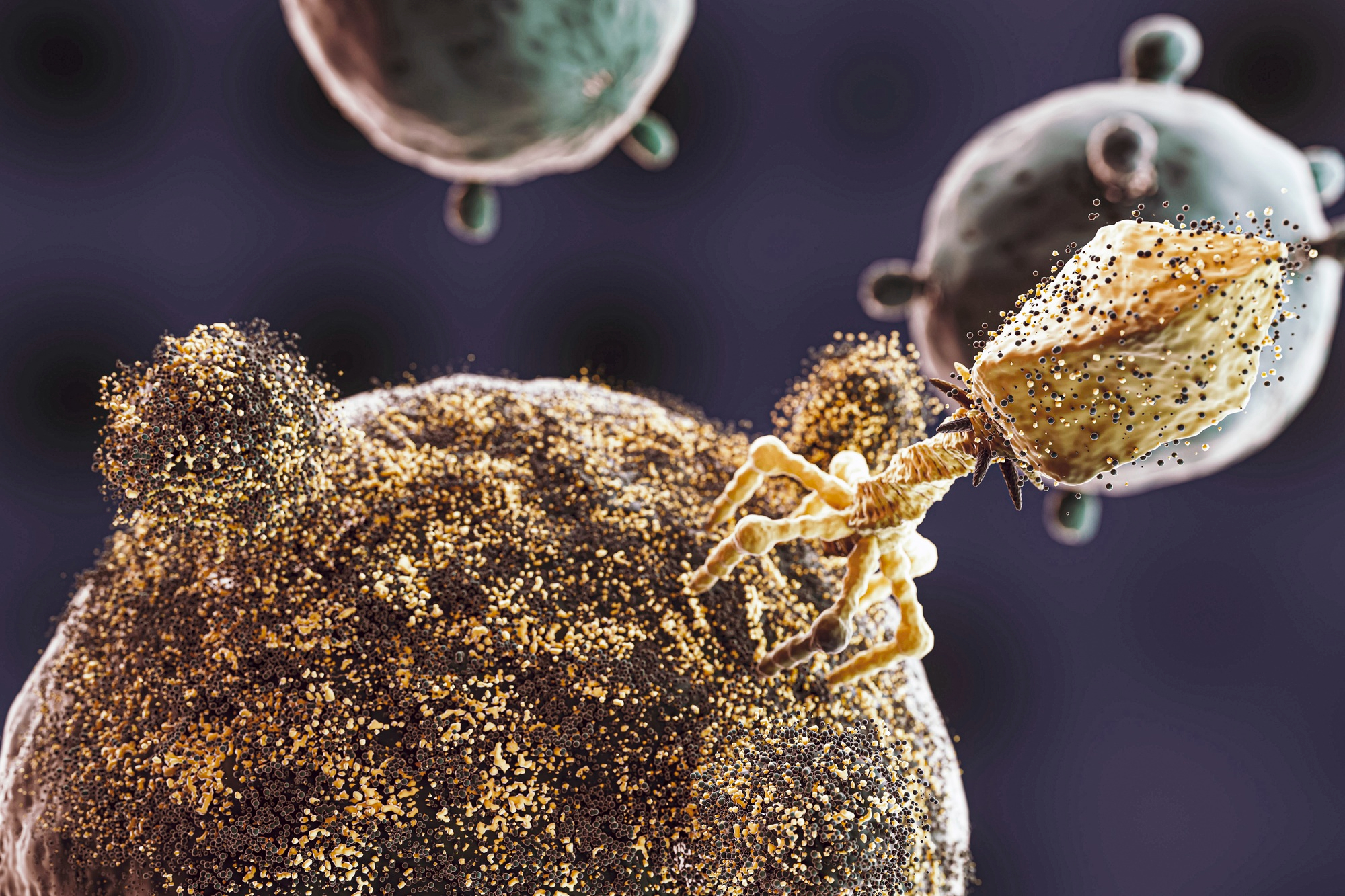
Bacteriophages: unique allies in the fight against AntiMicrobial Resistance
Bacteriophages, or phages, are viruses that specifically target and destroy bacteria. Ubiquitous in nature and found in high abondance, phages offer a promising solution to combat bacterial infections, particularly those resistant to antibiotics. Despite their long history in therapeutic application, especially in Eastern Europe, their potential remains underutilized globally due to various scientific, regulatory, and market challenges.
To advance phage-based therapies as medicinal products, addressing and overcoming key considerations will be important:
- Regulatory Frameworks: The development of Phage Therapy Medicinal Products (PTMPs) requires regulatory pathways tailored to the unique nature of phages. Unlike traditional drugs, phages are inherently diverse, necessitating innovative approaches to demonstrate their quality, safety, and efficacy. Regulatory bodies are beginning to adapt, but harmonized global guidelines are still in development.
- Adaptive Manufacturing: The variability of phages and their evolving targets call for flexible manufacturing processes. Producing phages consistently at a high quality, while ensuring scalability and compliance with Good Manufacturing Practices (GMP), remains a technical challenge that requires new solutions
- Clinical Validation: Phage therapies often involve personalized or semi-customized approaches, making it essential to design clinical trials that balance scientific rigor with practical feasibility. Innovative testing methodologies are essential to speed up validation and approval processes.
- Market Access Challenges: Phage therapies do not align with the traditional “blockbuster drug” model due to their specificity. To ensure their availability and affordability, collaborative efforts are needed to integrate phage therapies into evolving health technology assessment frameworks and reimbursement systems.
Our Approach
Founded in March 2024, the Phage Action Collaborative Taskforce (Phage-ACT) brings together stakeholders to advance the development and accessibility of Phage Therapy Medicinal Products (PTMPs) and Phage-derived products.
With members from academia, biotech industry, governmental and healthcare organizations, Phage-ACT focuses on:

Align efforts across regulatory bodies to create consistent guidelines and standards, reducing barriers to phage therapy development and access

Establish flexible frameworks for platform-based approaches that address phage diversity and enable rapid adaptation to evolving bacterial resistance

Define IVD regulatory pathways and address challenges related to standardization and reference methods to ensure reliable clinical use and compliance

Promote innovative trial designs tailored to bacterial targeting and personalized treatments to accelerate clinical validation

Collaborate with HTA bodies to align phage therapies with evolving market access pathways, ensuring pricing and reimbursement meet public health needs
BEAM Phage-ACT is freely accessible to all BEAM members.

