Diagnostics

Misuse, including overuse, of antimicrobials is one of the main drivers of AMR. Diagnostic tests can help inform prescription to prevent misuse. They can also ensure preparedness by generating timely surveillance data.
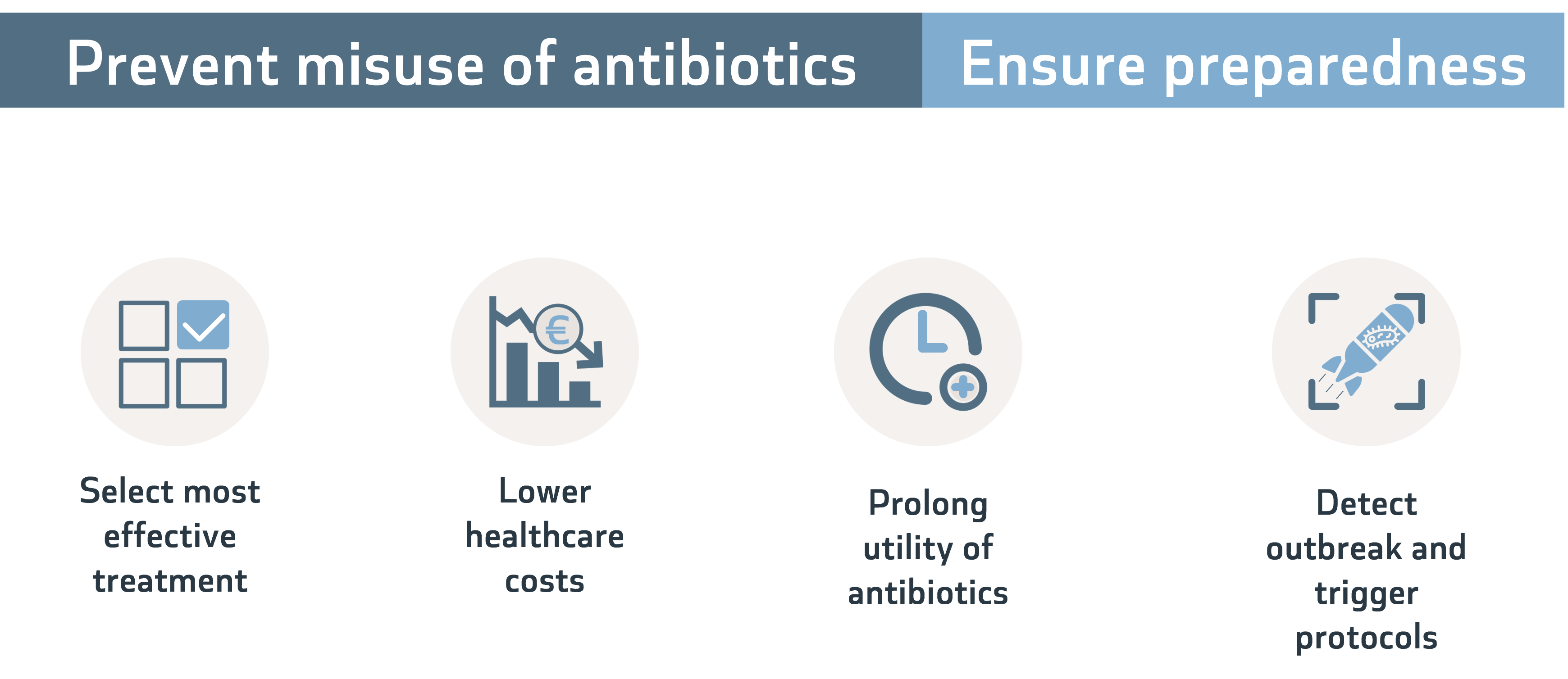
But developers face numerous roadblocks along the way from idea to market.
The full benefit of diagnostic test remains unrecognised by clinicians and payers, leading to limited acceptance and uncertain reimbursement paths.
The European Regulatory framework is also not helping, with the cumbersome IVD Regulation and the lack of centralised organisation, such as the European Medicine Agency for drugs or the Food and Drug Administration in the US.
Our Approach
The BEAM Taskforce on Diagnostics (BTF-Dx) provides a platform to discuss major issues faced by the AMR diagnostic community.
BTF-Dx gathers expertise, discusses topics of interest and conveys the SMEs’ messages to stakeholders and policymakers at EU and Member States level.
Various themes are covered.
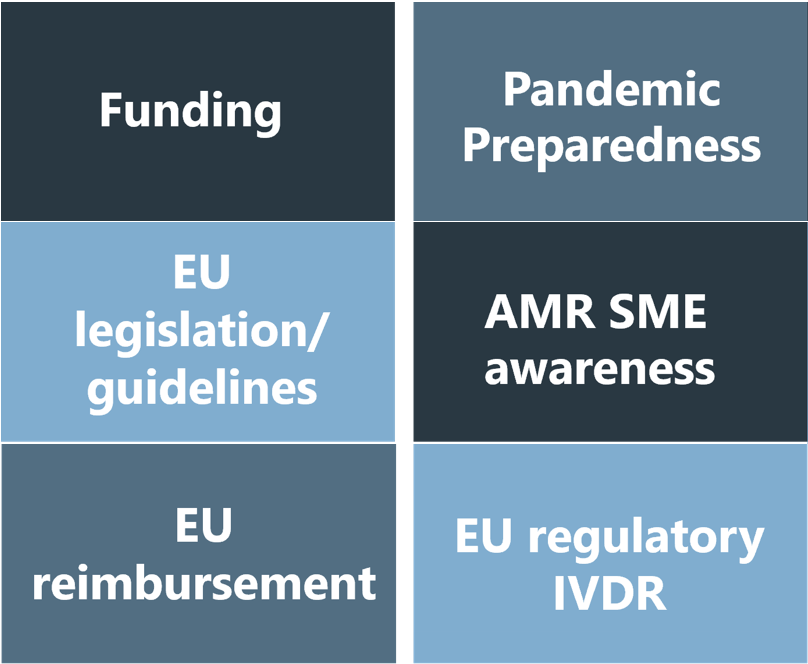
Messages raising diagnostics SMEs’ needs are developed through collective reflection, ad hoc surveys, brainstorming sessions, etc. External experts can also be involved on an ad hoc basis.
BTF-Dx is freely accessible to all BEAM members.

